Results
Subjects
- A total of 663 subjects were treated in the INBUILD trial.
Baseline characteristics of subjects in the INBUILD trial
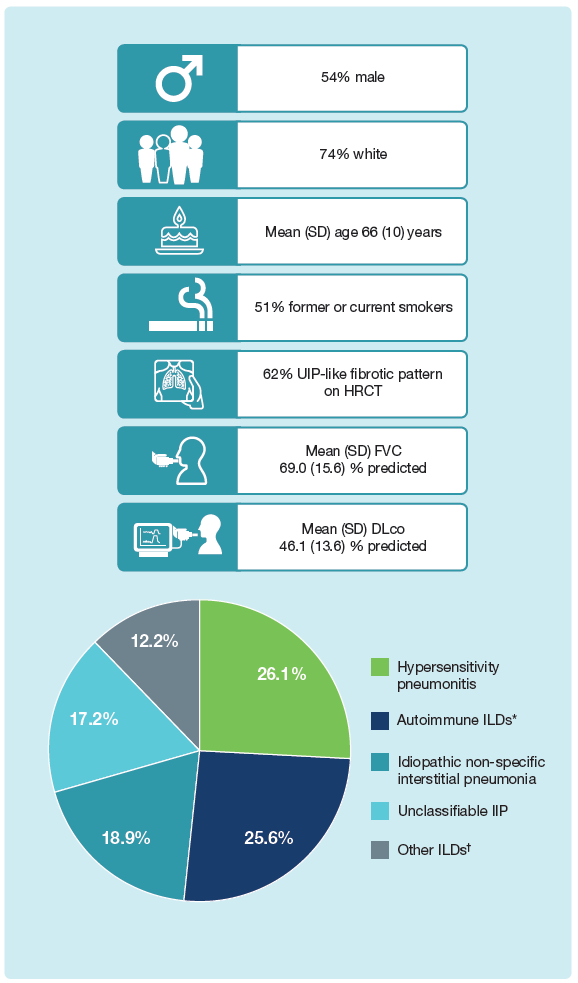
*Included RA-ILD, SSc-ILD, MCTD-ILD, plus autoimmune ILDs in “Other fibrosing ILDs” category of case report form.
†Included sarcoidosis, exposure-related ILDs and other terms in “Other fibrosing ILDs” category of case report form.
Categorical changes in FVC % predicted
- At week 52, 27% of subjects in the nintedanib group and 13% of subjects in the placebo group had an increase or no decline in FVC % predicted.
Absolute and relative declines from baseline in FVC at week 52
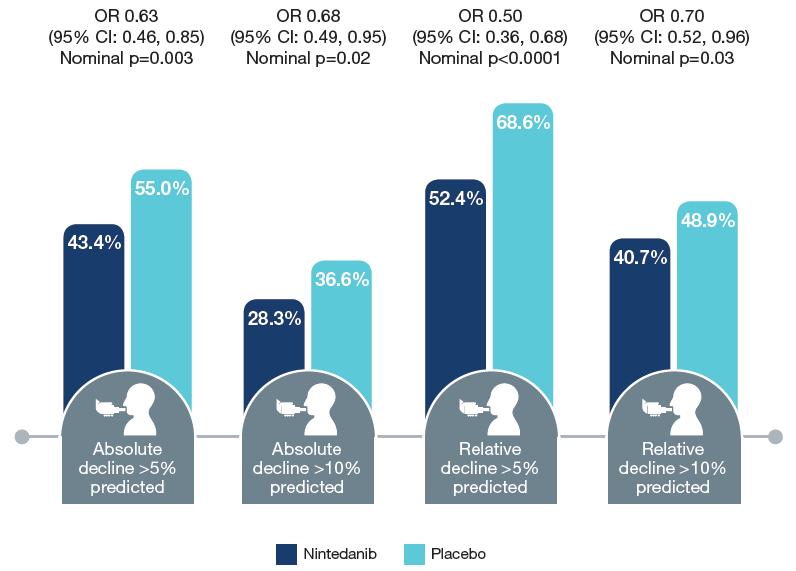
Percentages shown are the percentage of patients with the respective decline. Subjects with missing data were assumed to have had the respective decline (worst case analysis).
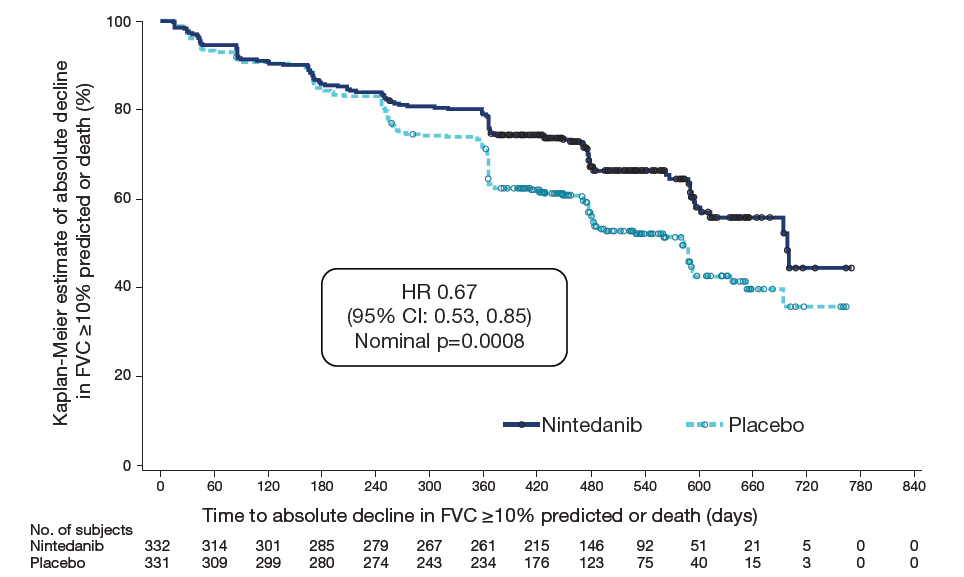
Time to absolute decline in FVC ≥10% predicted or death using data up to first database lock
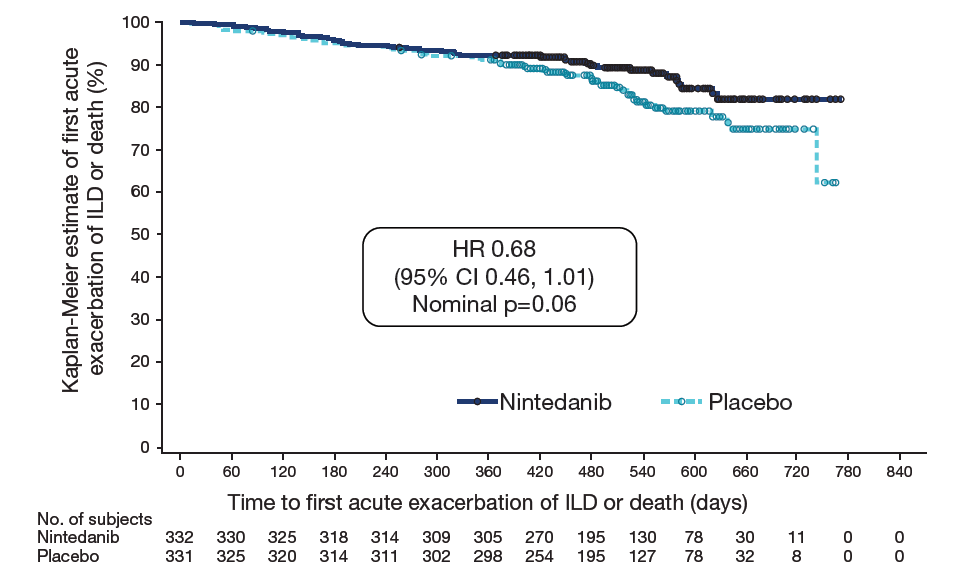
Time to first acute exacerbation of ILD or death using data up to first database lock