Results
Subjects
- In the nintedanib and placebo groups, respectively, 180 (62.5%) and 178 (61.8%) of subjects had extensive ILD.
Baseline characteristics of subjects with extensive and limited ILD
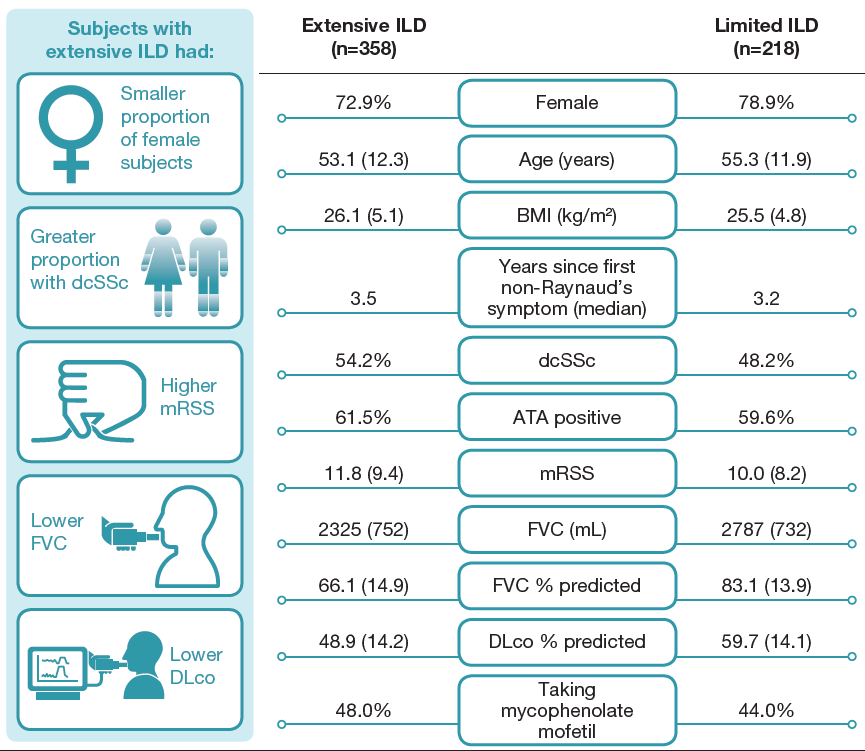
% of subjects or mean (SD) unless otherwise stated. ATA, anti-topoisomerase I antibody; dcSSc, diffuse cutaneous SSc; mRSS, modified Rodnan skin score.
Rate of decline in FVC (mL/year)
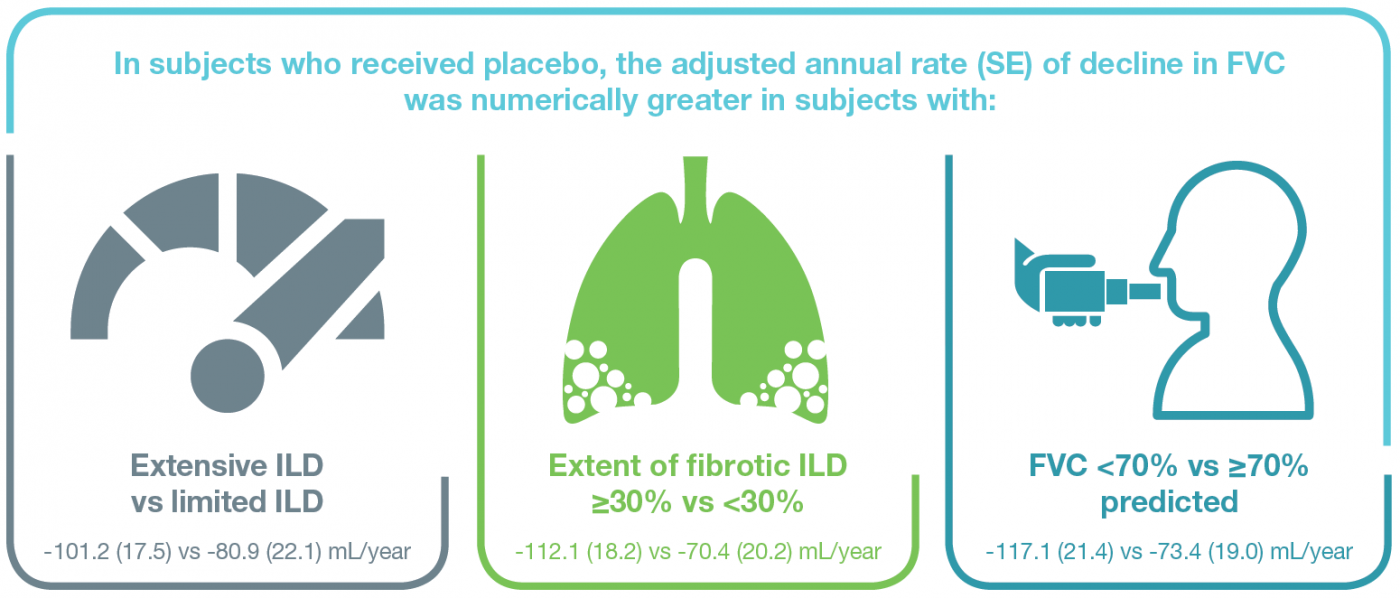
- The effect of nintedanib versus placebo on the rate of FVC decline was numerically greater in subjects with extensive than limited ILD, and in subjects with extent of fibrotic ILD on HRCT ≥30% than <30%, but the exploratory interaction p-values did not indicate heterogenous treatment effects between subgroups. The effect of nintedanib versus placebo was consistent between subjects with FVC <70% and ≥70% predicted at baseline (Figure 1).
Figure 1. Rate of decline in FVC (mL/year) with nintedanib versus placebo in subgroups by extent of ILD and FVC % predicted
Extensive ILD: extent of fibrotic ILD on HRCT >30%, or extent of fibrotic ILD on HRCT >10% to ≤30% with FVC <70% predicted.
Limited ILD: extent of fibrotic ILD on HRCT >10% to ≤30% with FVC ≥70% predicted.
Proportion of subjects who had absolute and relative declines in FVC, and who had an absolute decline in FVC ≥10% predicted or died, over 52 weeks
- No heterogeneity was detected between subgroups in the effect of nintedanib versus placebo on categorical declines in FVC (Figure 2).
Figure 2. Absolute and relative declines in FVC at week 52 in subgroups by extent of ILD
OR, odds ratio. Missing data were imputed using a worst value carried forward approach.
- Fewer subjects with limited or extensive ILD treated with nintedanib than placebo had an absolute decline in FVC ≥10% predicted or died over 52 weeks (Figure 3).
Figure 3. Proportion of subjects who had an absolute decline in FVC ≥10% predicted or died over 52 weeks in subgroups by extent of ILD
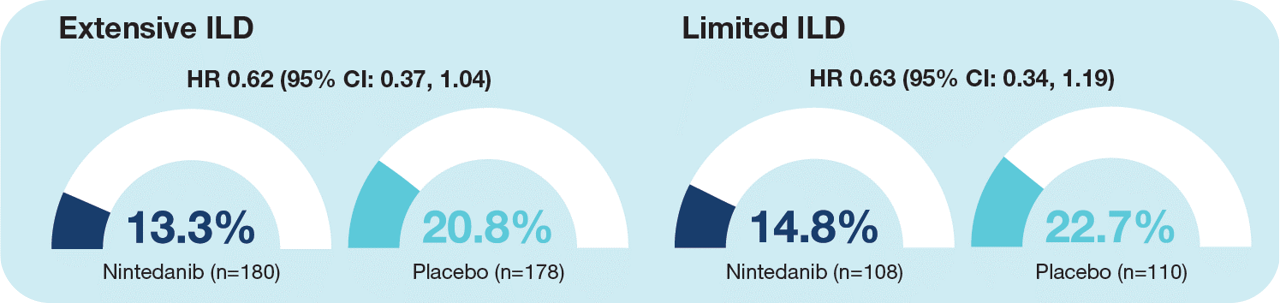
Treatment-by-subgroup interaction p=0.98. Missing data were imputed using a worst value carried forward approach.
Adverse events
- The adverse event profile of nintedanib was consistent between subgroups by extensive or limited ILD at baseline.
Adverse events
Data are n (%) of subjects with ≥1 such adverse event reported over 52 weeks (or until 28 days after last trial drug intake for subjects who discontinued trial drug before week 52). Adverse events were coded based on preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA). *Reported in >10% of the overall population. †Adverse event that resulted in death, was life-threatening, resulted in hospitalization or prolongation of hospitalization, resulted in persistent or clinically significant disability or incapacity, was a congenital anomaly or birth defect, or was deemed to be serious for any other reason.