Results
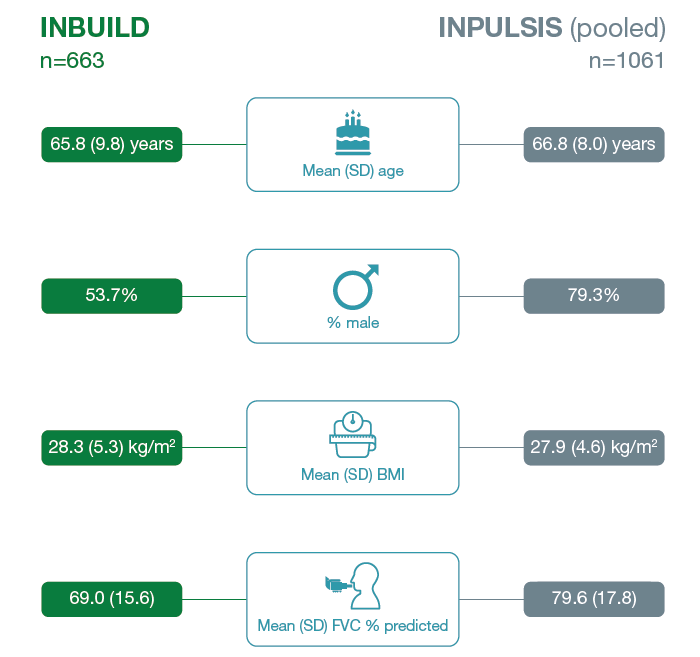
Baseline characteristics
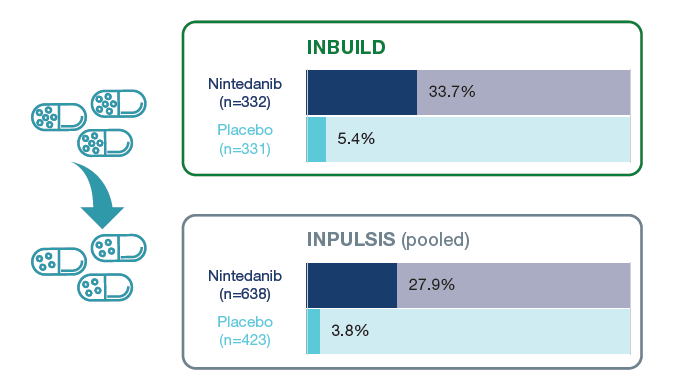
Proportions of patients who had ≥1 dose reduction over 52 weeks
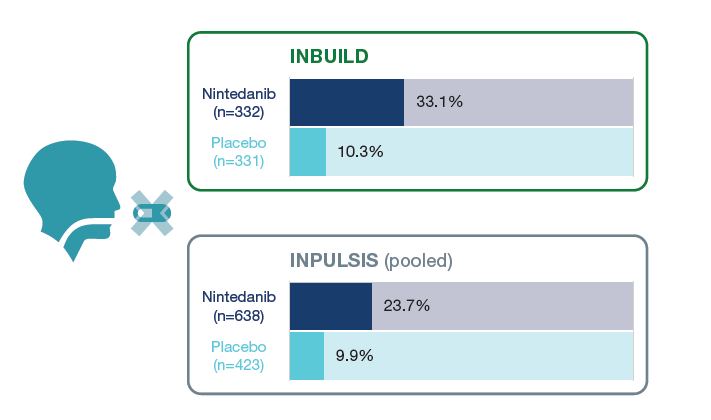
Proportions of patients who had ≥1 treatment interruption over 52 weeks
Mean dose intensity*
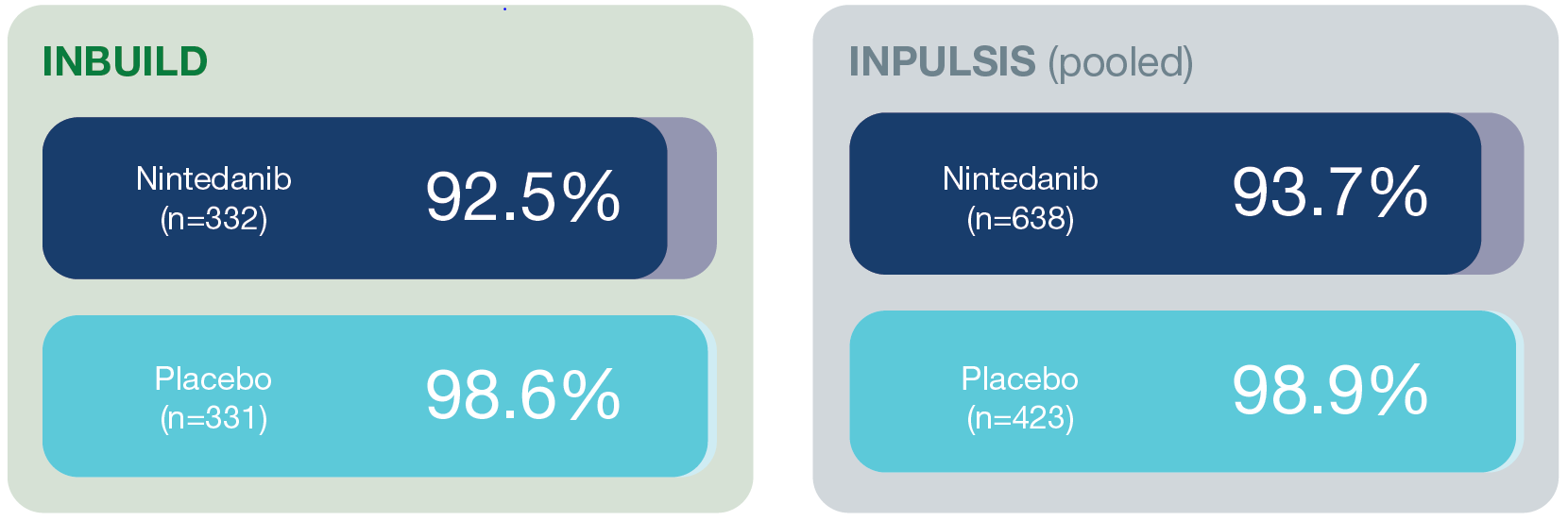
*Amount of drug administered divided by the amount of drug that would have been received if the 150 mg bid dose had been administered over the 52-week treatment period or until permanent treatment discontinuation.
Summary of adverse events
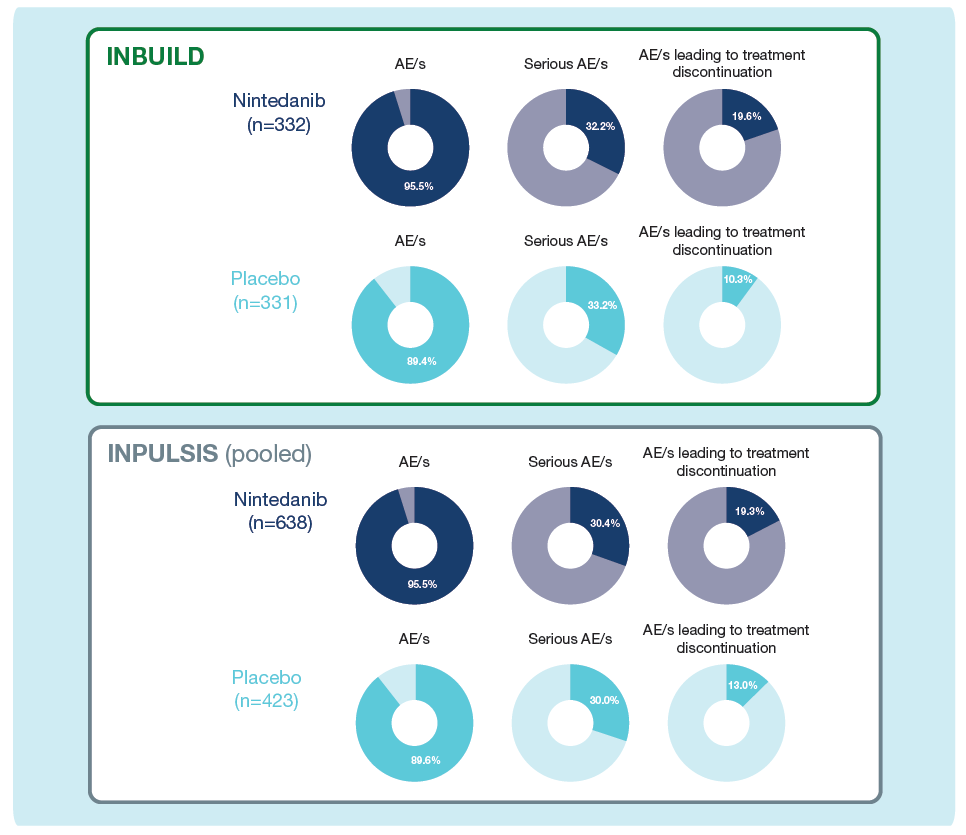
% of patients with ≥1 such AE reported over 52 weeks (or until 28 days after last trial drug intake for patients who discontinued trial drug before week 52).
Most frequent adverse events
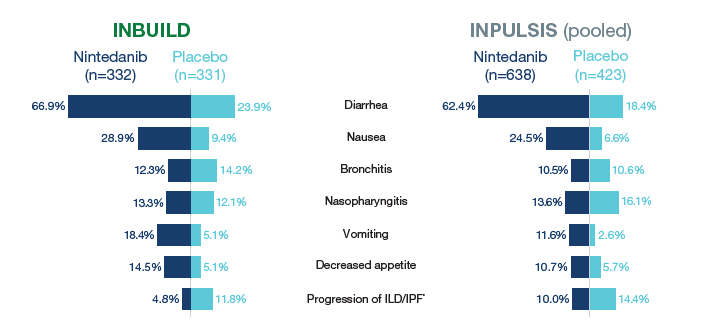
% of patients with ≥1 such AE reported over 52 weeks (or until 28 days after last trial drug intake for patients who discontinued trial drug before week 52). AEs were coded based on preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) (version 22.0 for INBUILD, version 16.1 for INPULSIS). AEs reported in >14% of patients in any treatment group are shown. *Based on MedDRA preferred term “interstitial lung disease” in INBUILD and “idiopathic pulmonary fibrosis” in INPULSIS.
Most frequent adverse events leading to permanent treatment discontinuation
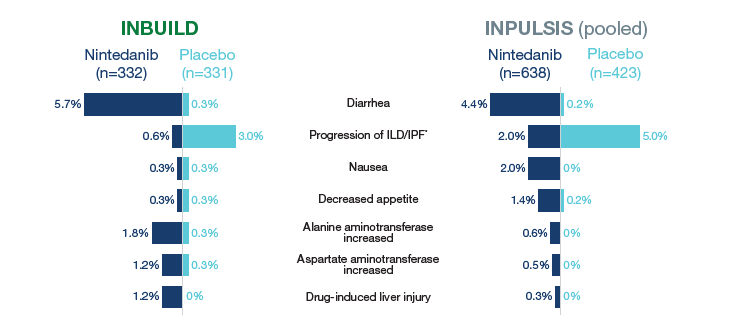
% of patients with ≥1 such AE reported over 52 weeks (or until 28 days after last trial drug intake for patients who discontinued trial drug before week 52). AEs were coded based on preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) (version 22.0 for INBUILD, version 16.1 for INPULSIS). AEs that led to treatment discontinuation in >1% of patients in any treatment group are shown. *Based on MedDRA preferred term “interstitial lung disease” in INBUILD and “idiopathic pulmonary fibrosis” in INPULSIS.
