Results
Patients
Baseline characteristics at inclusion in SENSCIS-ON
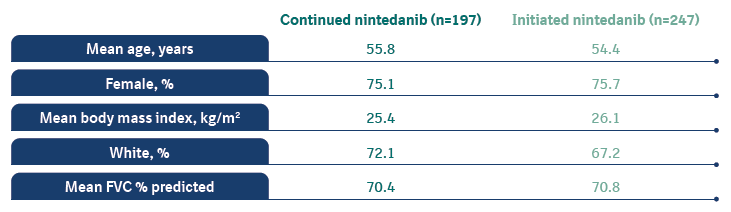
Mean (SD) FVC at the start of SENSCIS was 72.4 and 72.7 % predicted in the nintedanib and placebo groups, respectively.
Changes in FVC
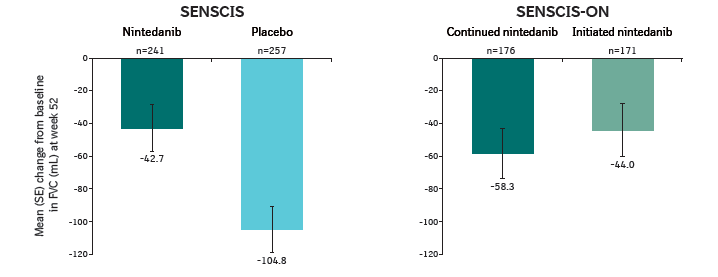
Change from baseline in FVC (mL) at week 52 in SENSCIS and SENSCIS-ON
Change from baseline in FVC (mL) in SENSCIS and SENSCIS-ON (pooled)
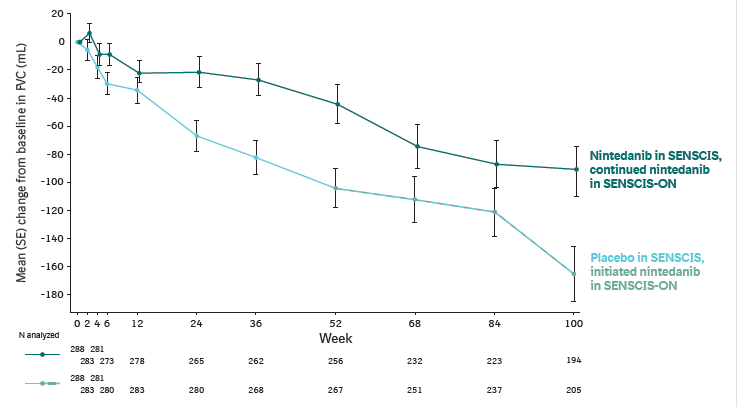
Patients from the drug–drug interaction study were not included in this figure.
Mean (SD) off-treatment period between SENSCIS and SENSCIS-ON was 47.2 (17.9) and 49.6 (19.3) days in patients who continued and initiated nintedanib in SENSCIS-ON, respectively. From week 52, data from SENSCIS-ON were included in both arms as patients transitioned into the open-label study. For patients who initiated nintedanib in SENSCIS-ON, the duration of placebo treatment in SENSCIS was 52 to 100 weeks and the duration of nintedanib treatment in SENSCIS-ON was 0 to 48 weeks.
Proportions of patients who met proposed thresholds for worsening of FVC, stable FVC and improvement in FVC from baseline of SENSCIS-ON to week 52 of SENSCIS-ON
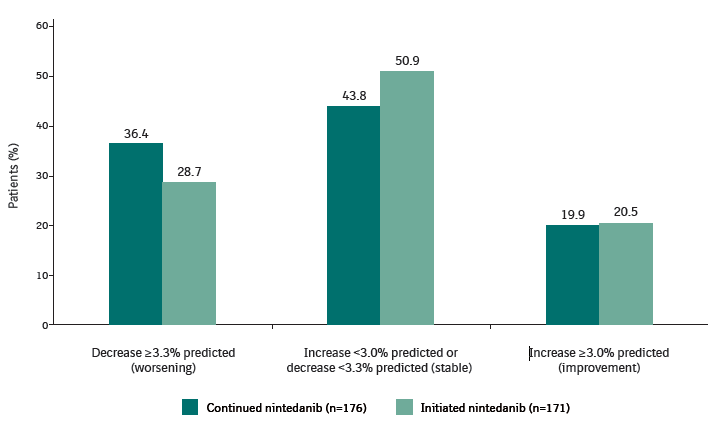
Proposed thresholds for worsening of FVC, stable FVC and improvement in FVC based on data from Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Short Form-36.2
Adverse events
Adverse events (reported irrespective of causality) over 52 weeks in SENSCIS and SENSCIS-ON
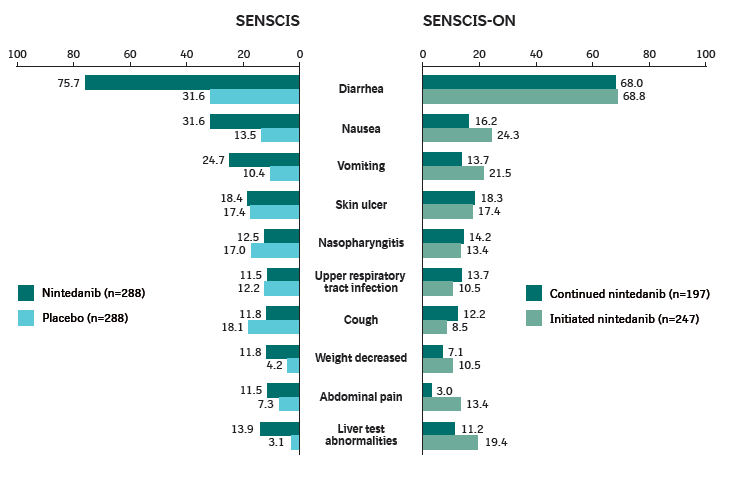
Adverse events were coded according to preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) except for “liver test abnormalities”, which was based on the standardized MedDRA query “liver related investigations, signs and symptoms” (broad definition). Data are % of patients with ≥1 such event reported over 52 weeks (or until 28 days after last drug intake if earlier in SENSCIS, or until 7 days after last trial drug intake if earlier in SENSCIS-ON). Events reported in >10% of patients in either group in SENSCIS-ON are shown.
Intensity of worst diarrhea event over 52 weeks in SENSCIS-ON
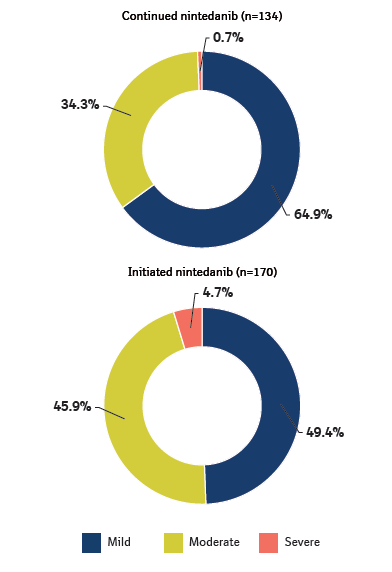
Data are % of patients who had ≥1 diarrhea adverse event over 52 weeks (or until 7 days after last trial drug intake for patients who discontinued trial drug before week 52).
Dose adjustments and treatment discontinuations over 52 weeks of SENSCIS-ON
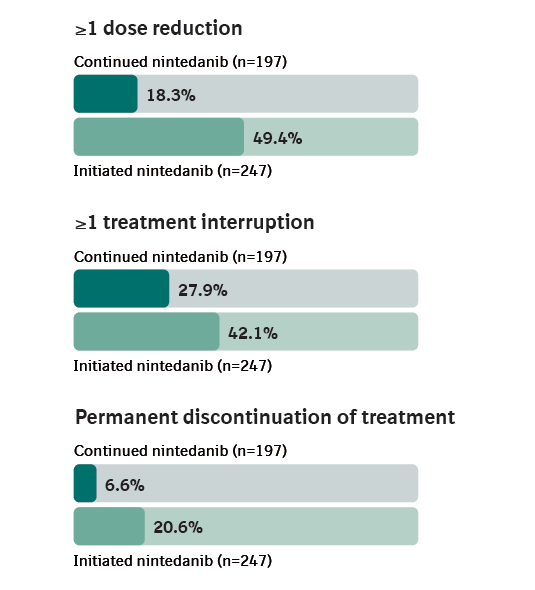
Permanent discontinuations were assessed up to week 52.