Methods
- This study (clinicaltrials.gov NCT04316780) included patients with IPF listed for lung transplantation between 1 July 2015 and 30 June 2019, who underwent lung transplantation and had been treated with nintedanib or pirfenidone continuously for ≥90 days at the time of listing for transplantation. Patients who underwent additional interventions (e.g. coronary artery bypass grafting, valve replacement) at the time of their lung transplant were excluded.
- We used data from medical records to assess complications during and in the 6 months after transplant in three groups of patients, based on the time between discontinuation of anti-fibrotic medication and transplant:
.
Image
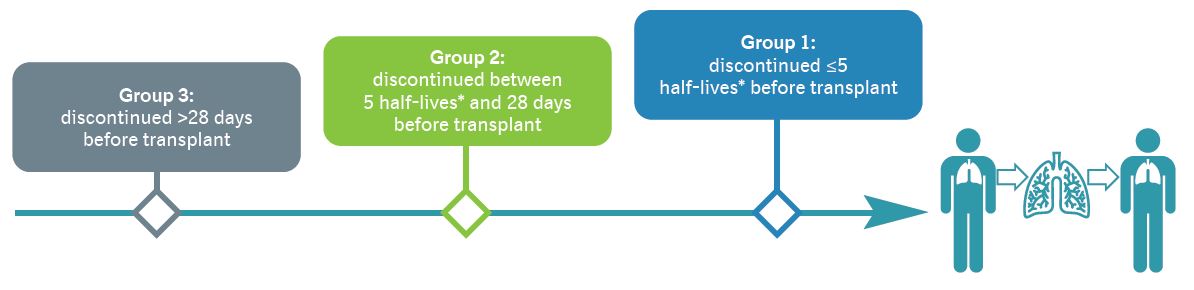
*≤2 days for nintedanib and ≤1 day for pirfenidone
- Analyses were descriptive.